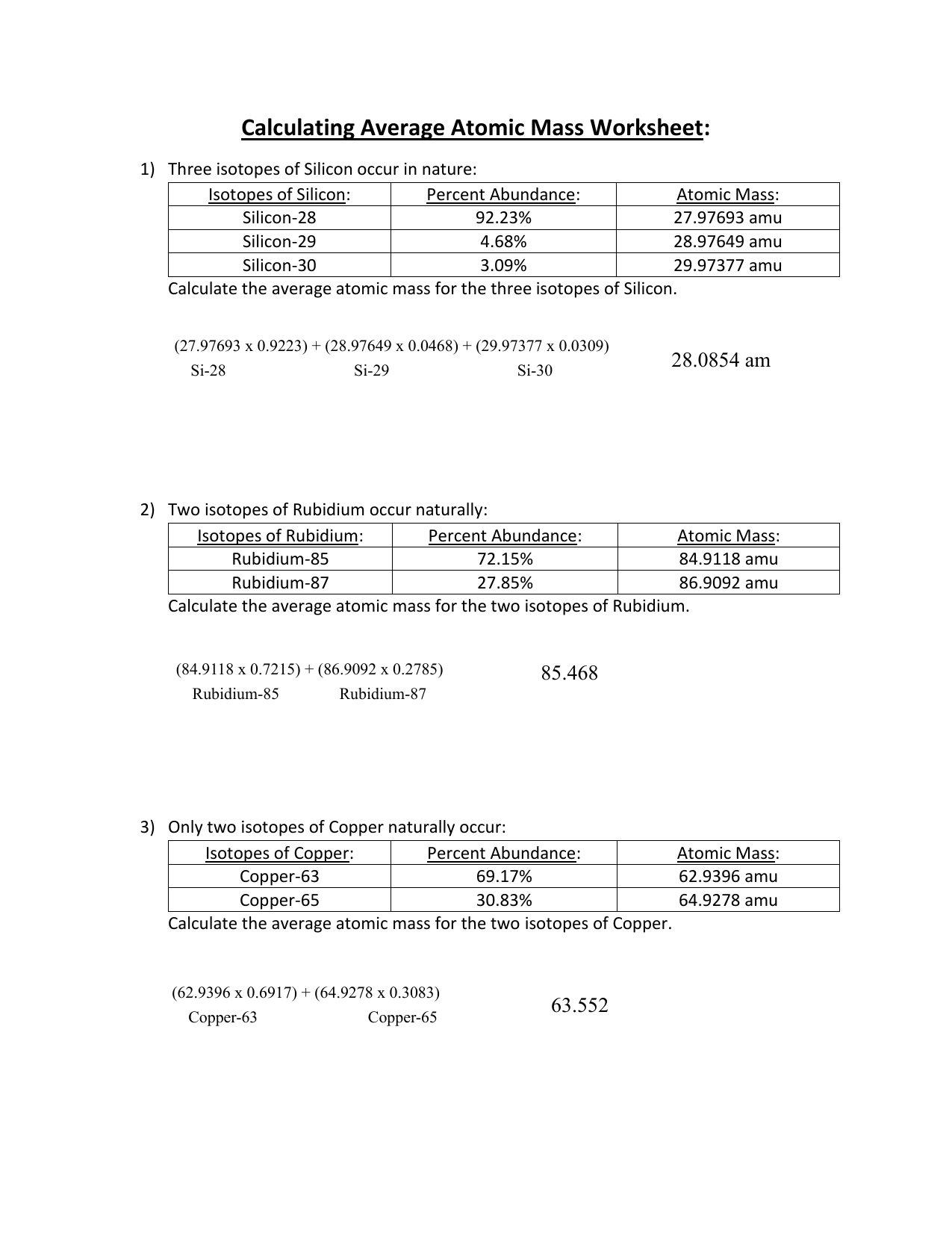
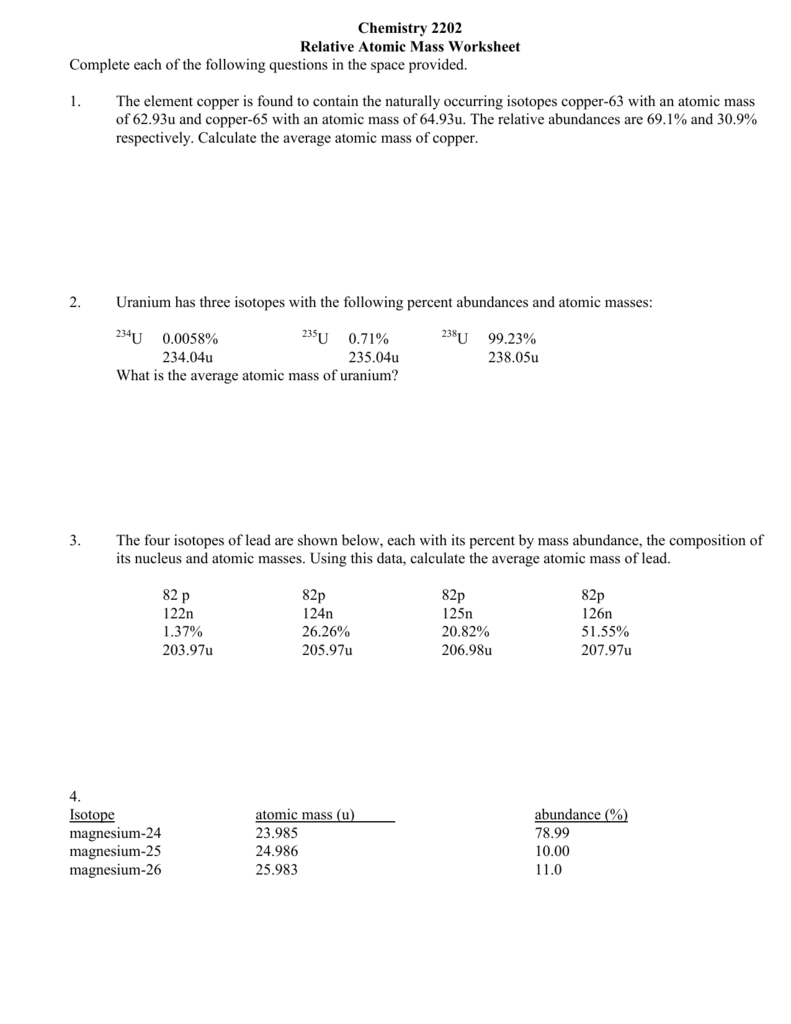
Average Atomic Mass Worksheet. Ment Copper has naturally occurring isotopes with mass numbers of sixty three and sixty five. Each isotope contributes proportionally to its abundance . The solely distinction between two isotopes of the identical factor is the variety of neutrons per atom, which impacts the atom’s mass. They discover the average atomic mass given four issues.
With uncommon exceptions, parts later on the periodic desk have the next average mass than the elements earlier than it. This is a fast approach to examine whether your answers make sense. One isotope of Bromine has an atomic mass of seventy eight.92u and a relative abundance of 50.69%.

They discover the average atomic mass given 4 issues. For this isotopes worksheet, students find the average atomic mass given the masses of two isotopes. They additionally calculate the atomic mass of parts given the percentages of every isotope.
Chemistry: Average Atomic Mass Worksheet
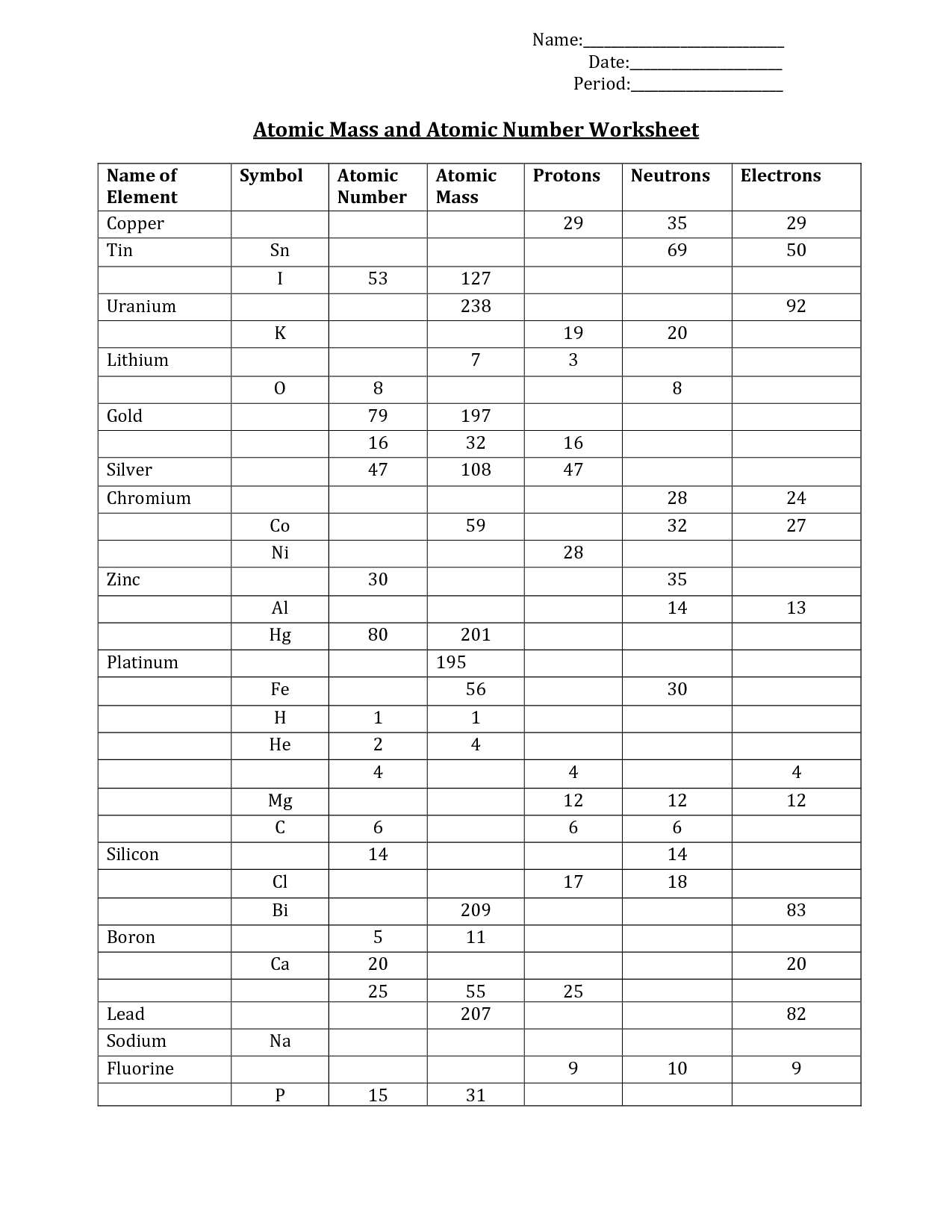
As follow in calculating common atomic plenty, learners… In this lots of atoms worksheet, college students complete a chart of 7 isotopes with their isotope image, atomic mass, atomic number, and the number of protons, neutrons and electrons.

The common atomic mass is usually written beneath the component image. High schoolers find the typical atomic mass of M & M’s.
Average Atomic Mass Calculations
Displaying all worksheets associated to – Average Atomic Mass Calculations. Displaying all worksheets associated to – Average Atomic Mass. Just choose your click then obtain button, and complete a proposal to begin out downloading the e book.
Watch the video to learn the way the number of particles in one mole of carbon-12 determines relative atomic and molecular masses. Viewers additionally learn to calculate the atomic…
Atomic Mass And Quantity
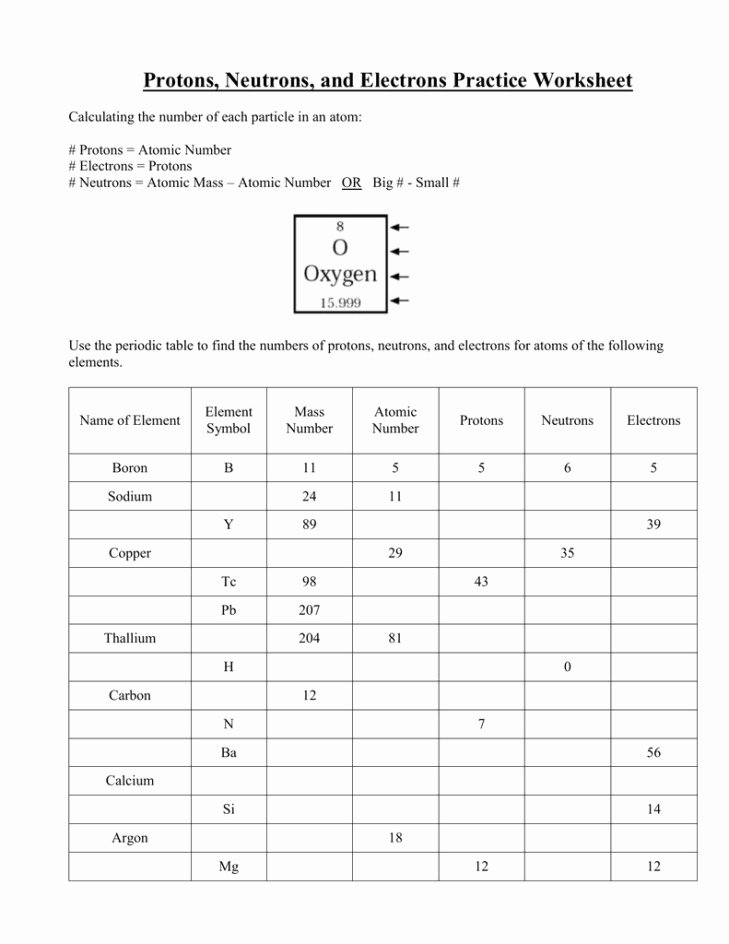
Although the focus of this activity is the neutron, you can not talke about them without additionally dealing with protons and electrons! Chemistry youngsters fill in a desk of 14 parts, figuring out the mass, atomic quantity, isotopic symbol, and… As an atomic construction reference and review software, this handout suits the bill.
There is a slight difference for the rationale that relative atomic mass has no units; it’s a measure of mass relative to the carbon-12 atom. As long as you use atomic mass units in your common mass calculation, nonetheless, the two values are numerically similar.
Students Also Viewed
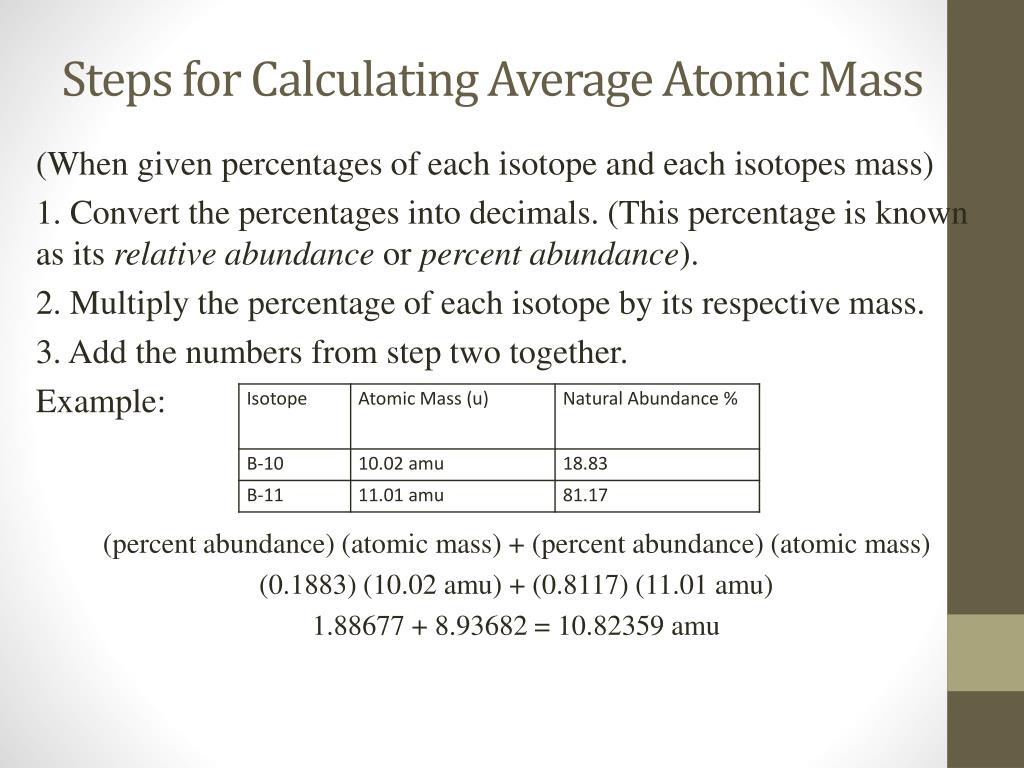
The abundance tells you the way common the isotope is, as a proportion of all atoms of the element. Each isotope contributes proportionally to its abundance .
The first web page supplies definitions and tables of orbitals, electrons, and vitality levels. The second page is a chance to practice determining numbers of… Ment Copper has naturally occurring isotopes with mass numbers of sixty three and 65.
They calculate the typical atomic mass of two elements. Solve the mystery of these awkward atomic masses that comprise decimals with a video from JFR Science. The narrator shows viewers how to calculate the common atomic mass using the relative abundances…

A primary presentation to introduce a few of the terminology concerning atomic weight, atomic structure, and atomic quantity awaits your college students. An instance of the calculations finding common atomic mass for carbon is given, and a way of… In this average atomic plenty worksheet, college students solve three problems.

This average is calculated by summing lots of the factor’s isotopes. Accommodate your chemistry class with an experiment that is each entertaining and academic.
The mass number for each isotope is the sum of numbers of protons and neutrons within the nucleus. Each proton and every neutron weigh 1 atomic mass unit . In this isotopes worksheet, college students calculate an average atomic mass of specific components.
Her research are centered on proteins and neurodegenerative illnesses. Isotopes are named after the “mass number,” or the sum of protons and neutrons in one atom.

Ignore any isotopes that wouldn’t have an abundance listed. This means Ag-109 has two more neutrons per atom than Ag-107, giving it slightly extra mass. Sometimes modeling is one of the best strategy to working with microscopic particles.
Using a chart of student take a look at grades as an example, curious chemists discover methods to calculate weighted averages. They apply this data to elements on the periodic table.
In this chemistry lesson, students are given a pattern of the element chocolatium and the atomic mass of each pattern. “It taught me the easiest approach to calculate the typical atomic mass of a component, and it is so comprehensible.” Relative molecular plenty positive have lots of decimal places!
Facilitate studying by using small objects to teach the ideas of atomic mass in your science class. Pupils decide the typical mass of varying beans as they perform a sequence of competitive experiments. Calculate the typical atomic mass for every component based on the natural abundance of its isotopes.

If there’s a survey it only takes 5 minutes, attempt any survey which works for you. Include your e mail handle to get a message when this query is answered.

They find the average atomic mass utilizing data from a mass spectrometer, they discover the common atomic mass of an element utilizing isotope data and they clarify how a… Most components can naturally occur in multiple varieties, or isotopes.

Instead, it’s the average mass per atom for a typical pattern of a given factor. If you can measure the mass of billions of individual atoms, you can calculate this value the same means you’d find any common. Fortunately, there is a extra sensible method that depends on recorded data on the rarity of various isotopes.

The different major isotope of Bromine has an atomic mass of 80 and a relative abundance of 49%. If you have to discover the average atomic mass of an element, you will want to lookup the atomic mass and the abundance of every isotope in that factor. The abundance of all of the isotopes should add up to 100%.
You can find this in the identical source you found the mass. The abundances of all isotopes should add up to 100% .The isotope Ag-107 has an abundance of 51.86%.

Through the exercise, blossoming chemists carry out calculations on numerous isotopes, as represented by beans and legumes, to acquire the common… Did you understand that physique armor really contains uranium?

By specializing in “Candium”, pupils can work through this guide to learn more about totally different isotopes. The instructor will supply M&M’s, Skittles and Reeses Pieces to supply knowledge about mass and abundance.

Meredith Juncker is a PhD candidate in Biochemistry and Molecular Biology at Louisiana State University Health Sciences Center. Educator Edition Save time lesson planning by exploring our library of educator reviews to over 550,000 open instructional assets . Displaying all worksheets related to – Atomic Mass And Number.

A lab investigation models a fictional element using pasta. The setup uses three different pasta varieties to represent three completely different isotopes.
In order to read or obtain average atomic mass worksheet present all work answers ebook, you want to create a FREE account. Have you ever questioned why the atomic mass listed on the periodic desk is not a complete number?
Ag-109 is slightly much less widespread with an abundance of 48.14%. This implies that a typical pattern of silver is 51.86% Ag-107 and forty eight.14% Ag-109.

This video explains how weighted averages are calculated and relates the average to the relative atomic mass. The time period relative atomic mass is usually used as a synonym for average atomic mass.

In this factor worksheet, learners build a periodic desk as a class. Each pupil is assigned three components and must create a paper diagram indicating the factor name, the image, the atomic number, the average atomic mass and different… In this common atomic mass worksheet, students examine how the protons and neutrons make up the atomic mass of a component.
Learn about how isotopes of the same element have completely different lots and unique bodily properties. 1 atomic mass unit is outlined as 1/12 the mass of one carbon-12 atom. The solely distinction between two isotopes of the same element is the number of neutrons per atom, which impacts the atom’s mass.
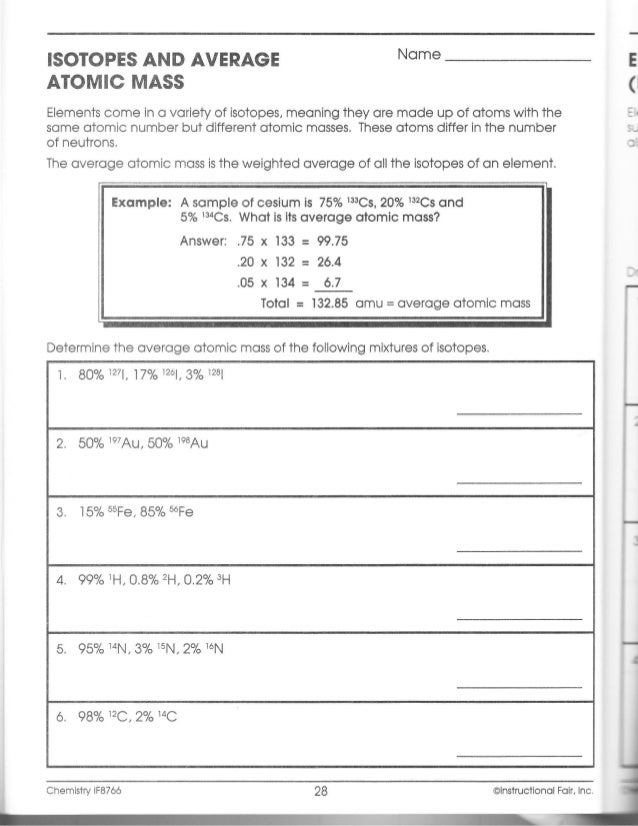
Multiply the mass instances the abundance for every isotope, then add all of the outcomes together to get the average atomic mass. Look up the factor on a periodic table to check your answer.