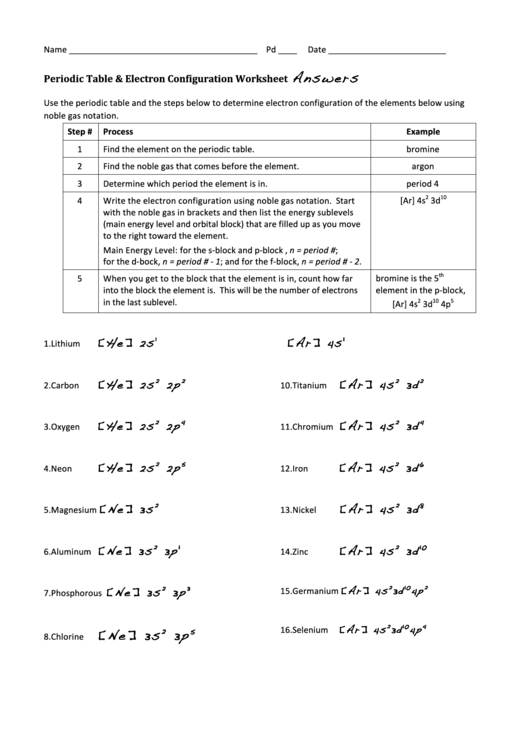
Electron Configuration Worksheet Answers. Balancing Equations Practice – A new version of Balancing Act that includes questions about subscripts and coefficients. However this has historically essentially the most commonly way of educating electron configurations. Links are supplied for the unit evaluation actions and different labs that tie in with these ideas. Also bear in mind to state the precise time the author should take to do your revision.
Particles , where the number of particles per mole is named the Avogadro fixed. Molar focus is the quantity of a selected substance per volume of solution, and is usually reported in mol/dm3.
The Cathode ray experiment was a results of English physicists named J. During his experiment he discovered electrons and it is among the most necessary discoveries in the historical past of physics.
Train Electron Configurations Worksheet Electron Configurations
If any atom does not have octet configuration, then you need to fulfil the octet valence of every individual atom. 2) Choose any factor of your selection from the periodic table.
For this challenge, the scholars must discover components that match the clues on the varied playing cards. Balancing Equations Practice – A new model of Balancing Act that features questions on subscripts and coefficients. Bonding Basics Review Worksheet – A student worksheet reviewing oxidation numbers in addition to ionic and covalent bonding.
Makes Use Of Of Cathode Ray Tube
The chemistry laboratory stereotypically makes use of various forms of laboratory glassware. However glassware just isn’t central to chemistry, and quite a lot of experimental (as properly as applied/industrial) chemistry is completed without it. A. Dragoset, Atomic Weights and Isotopic Compositions (version four.1), 2015, National Institute of Standards and Technology, Gaithersburg, MD, accessed November 2016.

The crystal lattice construction of potassium chloride , a salt which is shaped due to the attraction of K+ cations and Cl− anions. ; if it is the identical as zero the chemical response is said to be at equilibrium. A response is claimed to be exergonic if the ultimate state is lower on the vitality scale than the initial state; in the case of endergonic reactions the situation is the reverse.
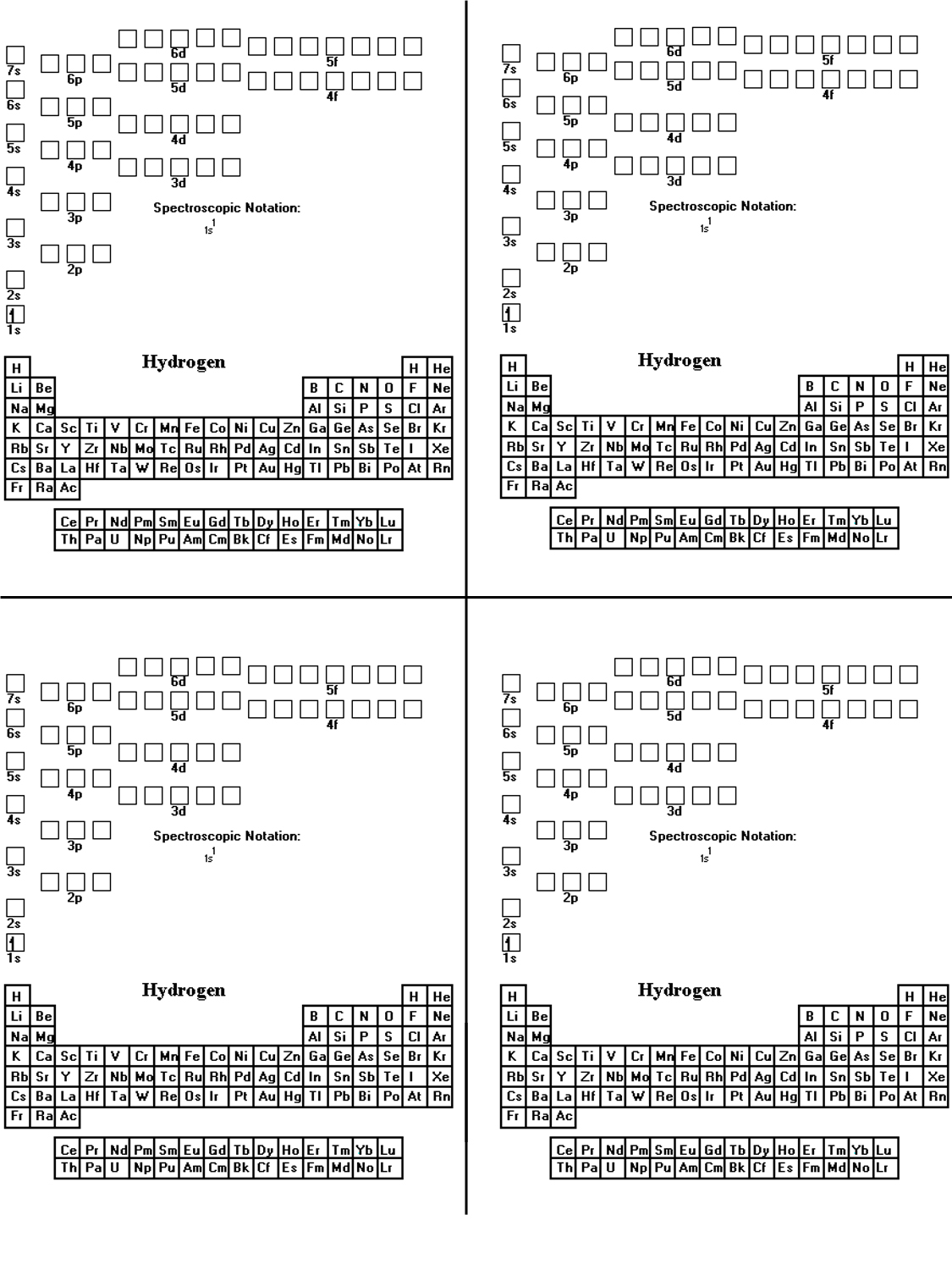
How To Attract An Electron Configuration Diagram
Hund’s rule denotes that electrons must occupy every single orbital of a subshell with no less than one electron with same spin course. And then they’ll start double occupying of orbitals of subshell. These four atomic orbitals are present around the nucleus of an atom and characterize completely different energy states.

Thomson realized that the accepted mannequin of an atom didn’t account for the particles charged negatively or positively. J. J. Thomson designed a glass tube that was partly evacuated, i.e. all the air had been drained out of the building. He then applied a excessive electrical voltage at either end of the tube between two electrodes.
Thomson concluded that rays had been and are basically negatively charged particles present or shifting around in a set of a constructive charge. This principle additional helped physicists in understanding the structure of an atom. And the significant remark that he made was that the characteristics of cathode rays or electrons didn’t rely upon the material of electrodes or the character of the gas present within the cathode ray tube.
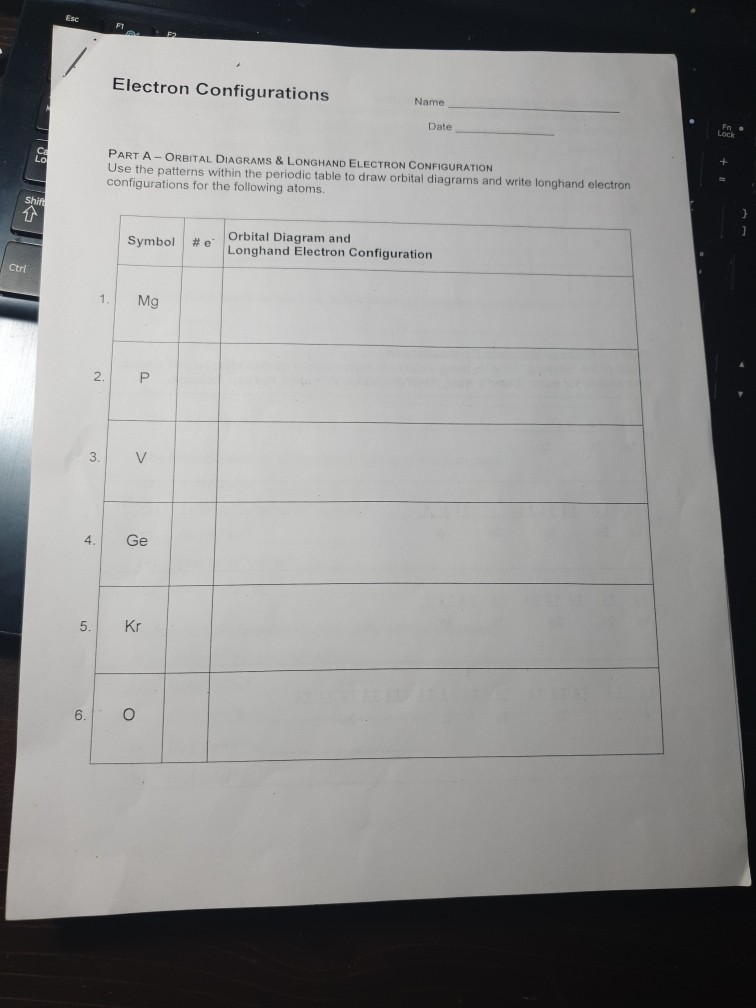
Electron Configuration Worksheet Reply Key
The number of electrons that are dispersed exterior the nucleus is the same because the number of positively charged protons in the nucleus. This explains the electrical neutrality of an atom as a complete. Most of the mass of the atom and all of its positive charge are contained in a small nucleus, known as a nucleus.

Since there’s 1 electron in the box labeled 1s, we are saying the H electron configuration in orbital notation is 1s1. Hydrogen is element 1 on the periodic table with 1 electron when it’s neutral. The idea is to draw an arrow for each electron, so on this case we just have one arrow to attract.
During this introductory lesson, students be taught the ideas behind balancing chemical equations. I instruct my students to make a listing of the atoms on each side of the equation to help them maintain monitor of their progress. As they add coefficients, they improve the numbers for those atoms of their lists.
The chemical conduct of atoms is controlled by their electron configuration. Since the families of components had been organized by their chemical conduct, it’s predictable that the person members of each chemical family may have comparable electron configurations. Thomson invented the electron by enjoying with a tube that was Crookes, or cathode ray.
The good news is that course assist on-line is right here to deal with all this wants to make sure all your assignments are accomplished on time and you have time for different essential actions. We also understand you might have a variety of topics to study and this might make it onerous for you to take care of all the assignments. You are expected to do an intensive research for each project to earn your self a great grade even with the restricted time you might have.

However this has historically the most commonly way of educating electron configurations. The mechanism by which this happens is defined in later lessons. Therefore electron configurations for ions must have electrons added or subtracted from the total.

For example lithium, sodium and potassium each have one lone valence electron, they all react violently when in contact with water to produce hydrogen gas. I developed this lesson to construct on the exercise calledElement Trading Cards and permit my students to explore the periodic properties of the Periodic Table of Elements.
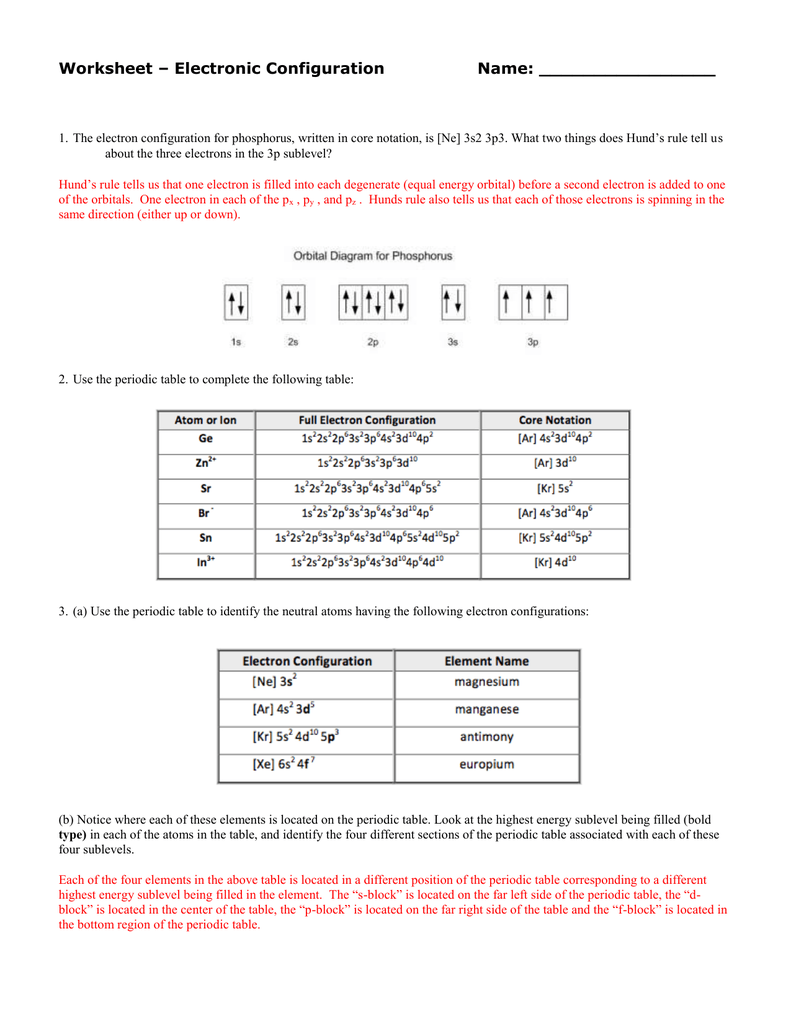
The on-line electron configurations worksheet above is designed to make it easy so that you simply can do. The 2, 8, eight, 18 rule is a really simplistic view of electron configuration and doesn’t give the full image in relation to electron configuration. While it really works for the primary 20 elements, in order to have a glance at other atoms greater than atomic number 20, we have to look closer on the types of orbitals in each electron shell in more detail.
For instance, the weather sodium (\(\ce\)) and magnesium (\(\ce\)) are both in period three. The parts astatine (\(\ce\)) and radon (\(\ce\)) are each in period 6.
The atomic quantity tells us the number of protons an atom possesses. In a impartial atom, the variety of protons and electrons are equal.
- An extra caveat is made, in that this definition includes cases where the interconversion of conformers is experimentally observable.
- With our course help on-line companies, you may be assured of a completely unique and error free paper written completely in your specified wants, directions and necessities.
- Many reaction intermediates with variable stability can thus be envisaged during the course of a response.
So, it becomes 1s2, 2s2, 2p6, 3s2, 3p6, 4s1, 3d10 or 4s1, 3d10. So one of the electrons from the 4s orbital is moved over to the 3d orbital. From right here, the third electron shell could have two electrons occupy its 3s orbital , followed by one other 6 electrons in its 3p orbitals .

Russian chemist Dmitri Mendeleev, creator of the periodic desk. 15th-century artistic impression of Jābir ibn Hayyān , a Perso-Arab alchemist and pioneer in organic chemistry.
We additionally don’t at any point resell any paper that had been beforehand written for a client. To ensure we submit original and non-plagiarized papers to our purchasers, all our papers are handed via a plagiarism check.

After putting 2 arrows within the first box called the 1s orbital and one other 2 arrows in the second field called the 2s, there are nonetheless 2 extra electrons to draw. After putting 2 arrows in the first field called the 1s orbital, there’s nonetheless one other arrow to draw.
This exponential dependence of a reaction rate on temperature is named the Arrhenius equation. The activation vitality necessary for a chemical response to happen may be in the type of warmth, gentle, electrical energy or mechanical drive within the form of ultrasound. Alchemy is commonly seen as linked to the hunt to turn lead or other base metals into gold, though alchemists had been also thinking about lots of the questions of contemporary chemistry.

Write the image in square brackets for the noble gasoline positioned at the far right on the horizontal row earlier than the component. Electrons occupy all of the completely different orbitals within the same sub-level before doubling up inside orbitals. The orbitals with lower energies, nearer to the nucleus are filled earlier than those with higher energies.

There exist solely limited potential states of power for electrons, atoms and molecules. These are determined by the principles of quantum mechanics, which require quantization of power of a sure system.
The following are some of the ways we make use of to ensure customer confidentiality. We also supply free revisions to our shoppers for assignments delivered. The free revision is obtainable within 7 days after the assignment has been delivered.

We even have a plagiarism detection system the place all our papers are scanned before being delivered to shoppers. In the Bohr mannequin, there are a quantity of rules that can help you draw accurate diagrams.

The second means is more algorithmic and doesnt actually provide a sense of the periodicity and the arrangement of electrons in components. The stage of issue is about the identical as the Periodic Table technique.
This quantum number is otherwise well-liked as orbital quantum quantity. The primary purpose of angular quantum number is to indicate the orbital shape and the type of subshell of an electron occupies. In order to learn or obtain Disegnare Con La Parte Destra Del Cervello Book Mediafile Free File Sharing e-book, you want to create a FREE account.

Generally, bodily adjustments do not contain the manufacturing of energy. A physical change involves very little to no absorption of power. The vertical columns on the periodic table are referred to as groups or households because of their comparable chemical habits.

Cathode rays are streams of electrons noticed in vacuum tubes (also known as an electron beam or an e-beam). If an evacuated glass tube is fitted with two electrodes and a voltage is utilized, it is observed that the glass opposite the unfavorable electrode glows from the electrons emitted from the cathode. The major contribution of this work is the model new strategy to modelling this experiment, using the equations of physical laws to describe the electrons’ motion with quite lots of accuracy and precision.

And welcome to Chemistry in its element, where we take a glance at the stories behind the weather that make up the world around us. This week, we’re persevering with our tour of the periodic table with a lung full of a gasoline that we received’t do with out.