Limiting Reactant Worksheet Answers. In order to read or obtain Disegnare Con La Parte Destra Del Cervello Book Mediafile Free File Sharing ebook, you want to create a FREE account. In practise, the precise yield, or quantity of product collected, is sort of always less than the theoretical yield. 6 moles NH3 eight moles NH3 12 moles NH3 82 moles NH3 seventy eight moles NH3 64 moles NH3. This worksheet has been left in its original format to permit for instructor addition and modification.
This worksheet has been left in its authentic format to allow for trainer addition and modification. The theoretical yield is the quantity of product that can be fashioned primarily based on the limiting reactant.
A complete, no prep worksheet that includes 8 issues to help college students grasp limiting and extra reactant problems with mass-mass stoichiometry thrown in. Check out the accompanying notes, worksheets, and assessment in my TPT store! Can accompany Modern Chemistry by Holt, Rinehart, and Winston textbook.
What Number Of Grams Of Water May Be Produced From 5 Fifty Five Grams Hydrogen And Four44 Grams Oxygen ?
So, Fe (3.35 mol) is the limiting reactant because it has the decrease worth as in comparability with O2 (5.6 mol). So, Sb (1.a hundred twenty five mol) is the limiting reactant as it has the lower value as compared to O2 (1.eighty three mol). A limiting reactant drawback is the place in the stoichiometric ratio of the reactants is not given.

A i 2 o 5 5 co 5 co 2 i g 280 g solution steps step 1 decide the moles of i 2 o 5 step 2 determine the moles of co step three do a limiting reagent check step four using the. Limiting reagent calculations are performed in the same method because the stoichiometric equations on Worksheet 11.
13 Moles Of Phosphorus P Reacts With 194 Grams Of Water H2o What’s The Limiting Reactant? P44 X 31Zero = 1240g
In practise, the actual yield, or amount of product collected, is kind of at all times less than the theoretical yield. The actual yield is typically expressed as a share yield, indicating what share of the theoretical yield was obtained. 6 moles NH3 eight moles NH3 12 moles NH3 82 moles NH3 seventy eight moles NH3 64 moles NH3.

Click the PDF to check the solutions for Practice Questions. Experts are tested by Chegg as specialists of their topic area.
If there is a survey it solely takes 5 minutes, try any survey which works for you. View Notes – Limiting Reagents and Percentage Yield Worksheet answers 1 from CHEM 214 at Old Dominion University.

The substance that is in excess that doesn’t get used up as a reactant. The reactant that is used up first and prevents more product from being made. Just select your click on then download button, and full an offer to begin downloading the ebook.
This worksheet is aligned to Unit-8 Stoichiometry for High School Chemistry. Students are required to finish 6 limiting reactant issues.

This worksheet offers ten examples for students to work through the processes of figuring out the limiting reactant, theoretical yield, and/or the % yield of a response. A full answer key is provided at the finish. This worksheet can be utilized in any Chemistry class, regardless of the students’ capability degree.

Pin On Printable Blank Worksheet Template. Limiting reagent percent yield practice worksheet.

Discover learning video games guided classes and different interactive actions for children. Determine the mass of iodine I2 which might be produced.
We are a gaggle of pros coming from different backgrounds and domains i.e. Engineering, Advance science & Research , Technology .

In other words, it determines the magnitude of the reaction. Limiting Reagent Worksheet All of the questions on this worksheet contain the following response. When this response is carried out in a given experiment solely 30.
Pin On Customize Design Worksheet Online. Calculate the quantity of C formed within the reaction 2A + 4B ⟶ 3C + 4D when 5 moles of A react with 6 moles of B.

Identify the limiting reagent, if any, within the following reaction mixtures. A reagent, also identified as an analytical reagent, is a substance or compound that’s added to a system to provoke or test a chemical response.

This reagent is the one that determines the quantity of product shaped. 9 Qualified Limiting Reactant And Percent Yield Worksheet Answers Di 2020.

This worksheet is for you — simply print and assess! You can use this resource in your physical science or chemistry unit as a formative or summative assessment.

So, P4 (15.5g) is the limiting reactant as it has the decrease value as compared to O2 (28.63g). So, H2O (28.8g) is the limiting reactant because it has the lower worth as compared to Al2S3 (31.17g). The reactant obtained with a smaller number is the limiting reactant.

It permits for the teacher and the coed to collect details about what they know and what they do not know. Do you want a worksheet to assist collect information about student understanding of the rules of stoichiometry to search out the limiting and excess reactants of chemical reactions?
Limiting reagents and share yield worksheet 1. Limiting reactant and proportion yield. Mole Conversion Problems Chemistry Dissected M…

Identify the limiting reagent within the production of NH3 in this state of affairs. In order to learn or obtain Disegnare Con La Parte Destra Del Cervello Book Mediafile Free File Sharing e-book, you have to create a FREE account. Since 1.5 is lower than 2.5, B is the limiting reagent as a outcome of it goes to be used first.

We hope this graphic will probably be one of wonderful reference. Worksheet 14 1 Worksheet 14 Limiting Reagents 1.

So, HBr (4.99g) is the limiting reactant because it has the lower value as in comparability with Al (9.737g). So, Cl2 (53.5g) is the limiting reactant because it has the lower value as in comparability with TiO2 (59.4g) and C .
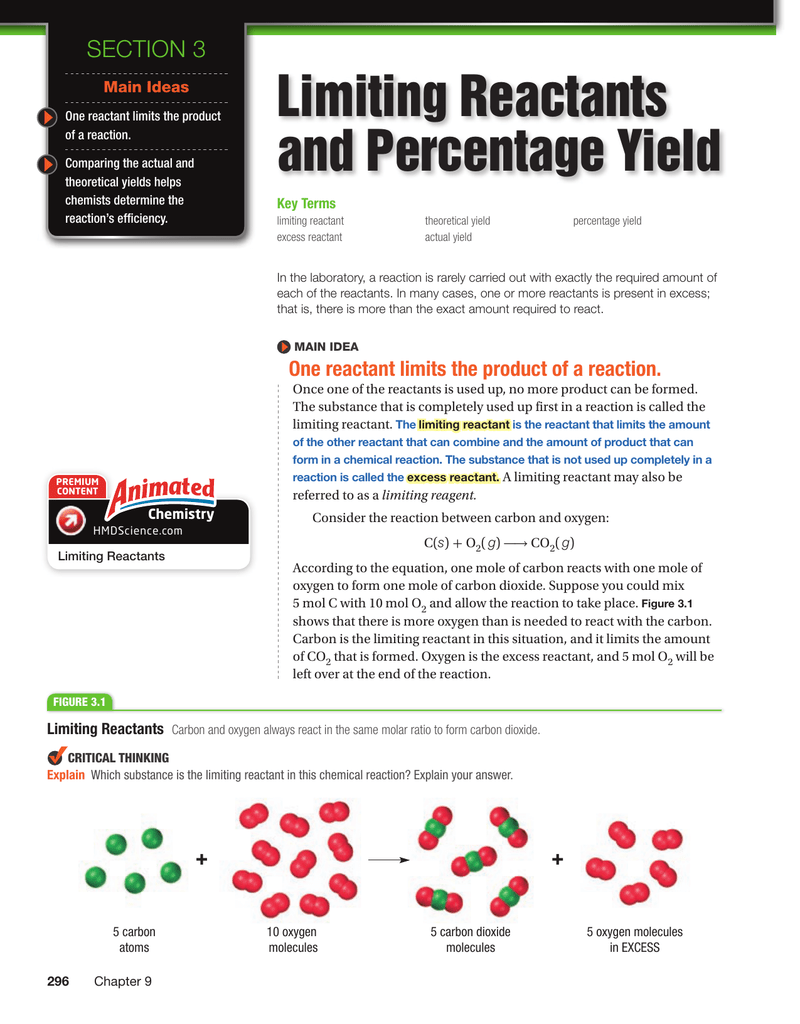
5 If 113 grams of sodium chloride are shaped within the response described in downside 2 what is the percent yield of this response. It was from reliable on line supply and that we love it. Compare the calculated amount of a reactant to the precise amount obtainable to determine which reactant is the limiting one.

So, O2 (0.004 mol) is the limiting reactant as it has the lower value as in comparability with NH3 (0.a hundred and five mol). So, H2O (6.7 mol) is the limiting reactant because it has the lower worth as compared to P4 .
We review their content and use your suggestions to maintain the quality high. Our library is the biggest of those which have literally lots of of hundreds of different merchandise represented. Displaying all worksheets related to – Limiting And Excess Reagents.

Percent Yield And Stoichiometry Notes And Practice Problems Worksheet Persuasive Writing Prompts Answer Keys Worksheets. This shows that 0.567 mol C2H3Br3 is required to react with all the oxygen. C2H3Br3 is the limiting reagent because there is solely 0.28 mol of it present.

In order to learn or download limiting reactant issues with solutions e-book, you have to create a FREE account. Ad Download over K-8 worksheets masking math reading social studies and more. Skillbuilder eight 6 Chemistry Worksheets Chemistry Education.

When it is consumed, the response will cease, regardless of the amount of reactant present in the response. It restricts the quantity of product produced.
In this limiting reactant issues what we determine is, there’s a reactant which limits the quantity of product that can be obtained or produced. Worksheets for all Download and Share Worksheets Free on. Step 4 utilizing the limiting reagent find the moles of i2produced.